parasite genus Schistosoma (which causes the disease Schistosomiasis) thrives in environmental and socio-ecological contexts that make transmission possible. Understanding the role of environmental factors is essential especially when linking control strategies to wholesale procurement of drugs such as mebendazole wholesale (anthelmintic procurement often discussed in broader helminth contexts). Below is a detailed discussion (≈ 1000 words) of how environmental factors shape Schistosoma infection spread, with reference to control implications (including drug availability) and how “mebendazole wholesale” may come into conversation in integrated helminth control programmes.
Transmission cycle and environmental fundamentals
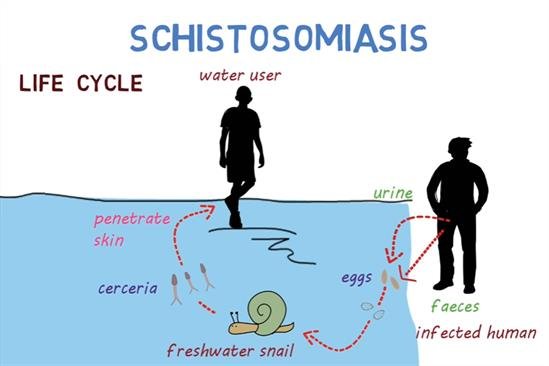
Schistosoma spp. require a freshwater snail (intermediate host) to complete their lifecycle. Humans (or animal reservoirs) release eggs via urine or faeces into freshwater, miracidia hatch and infect specific snail species; the snails release cercariae into water, which penetrate human skin on contact.
Key environmental elements
- Presence of freshwater bodies (lakes, ponds, slow-moving rivers, irrigation canals) where snails live.
- Suitable snail hosts (e.g., genera Bulinus, Biomphalaria, Oncomelania) whose ecology is determined by water temperature, turbidity, vegetation, pH and other water‐chemistry factors.
- Human exposure to infested water through domestic use (washing, bathing), occupational exposure (farming, irrigation, fishing), or recreational contact.
- Contamination of water with eggs through inadequate sanitation, open defecation/urination near water.
- Thus environmental factors are not just “nice to have” but are central to establishing and maintaining the transmission cycle of Schistosoma.
Key environmental and climatic influences
Several specific environmental factors influence how prevalent and intense Schistosoma infections become in a given community.
Water body characteristics & snail ecology
Surface water availability and permanence: Long‐standing, stable freshwater bodies allow snail populations to establish and sustain. For example, drought or ephemeral streams reduce snail habitat and thus transmission.
Water temperature, pH, salinity, turbidity, vegetation: These affect snail metabolism, parasite development inside snails, and cercarial shedding. For instance, warm water near optimal temperature can increase snail reproduction and parasite shedding.
Hydrological changes: Irrigation systems, dams, reservoirs and water‐resource development can expand snail habitat or bring humans into novel water contact. For example, the creation of man‐made reservoirs has in some settings increased risk of schistosomiasis by providing snail habitat.
Climate and weather patterns
Rainfall and flooding: Rain can expand wetland areas, increase snail‐habitat surface, and increase human-water contact (floodwaters, new riparian margins). A scoping review noted that floods contribute to higher snail infection rates (around 0.5 based on one metric).
Temperature shifts: As the climate warms, areas previously too cold for snail hosts or for parasite development might become suitable, thus extending the geographic range of schistosomiasis. But conversely, if temperatures exceed the snail’s thermal optimum, snail populations may decline.
Seasonal dynamics: In many endemic areas, transmission fluctuates with seasons (dry vs wet), affecting snail abundance, human water‐contact behaviour, and thus infection risk.
Sanitation and human behaviour
Inadequate sanitation: When open defecation or urination happens into or near water bodies, eggs get into the water and perpetuate the cycle.
Human contact behaviour: Who uses the water, how often, at what body surface exposure children playing, women washing clothes, farmers irrigating all contribute to risk. The more frequent the contact and the larger the exposed skin surface, the higher the chance of penetration by cercariae.
Socioeconomic factors: Poverty, lack of alternative clean water sources, reliance on natural water bodies, low awareness all interplay. For example, communities without piped water may rely on ponds for domestic chores, increasing exposure.
Spatial / ecological heterogeneity
Transmission is highly focal: even within endemic villages, micro‐habitats of snails, water‐contact points, human behaviour create hotspots.
Reservoir hosts: In some places, animals (cattle, wild mammals) can maintain schistosome species (especially zoonotic ones), linking animal grazing areas, water contamination, and human exposure.
Spread of Schistosoma infections via environmental change
Because these environmental factors are dynamic, several phenomena drive spread (or potential spread) of schistosomiasis.
Water‐resource development: New dams, irrigation schemes, drainage channels create novel habitats for snails or expand human‐water contact. As noted in older work: “man-made reservoirs and irrigation systems … have contributed to the spread of schistosomiasis.”
Population movements / migration: People moving into endemic zones, or snails being transported (e.g., via aquatic plants), can introduce the parasite into new areas.
Climate change and new geographical risk zones: As rainfall, temperature, and humidity patterns shift, areas previously low‐risk may become suitable for snail hosts and thus transmission. For example, the scoping review indicated new hotspots might emerge in Southeastern Asia, Pacific Islands, Mediterranean region due to environmental changes.
Environmental degradation and sanitation failure: Increased pollution, soil erosion, flooding, siltation of waterways can change snail habitat (sometimes positively, sometimes negatively) and change human-water contact patterns. For instance, soil contamination with eggs that wash into water.
So, environmental change is not static; it directly influences the potential for spread of schistosomiasis and the emergence of new endemic foci.
Implications for control and the place of drug procurement
Given that environmental factors are so central, control strategies must go beyond just treating people. They must include environmental management, snail control, sanitation improvements and behaviour change. Models show that treatment alone cannot sustainably eliminate schistosomiasis without tackling the environment.
From the drug‐procurement perspective (for example, mebendazole wholesale), although mebendazole is primarily used for soil-transmitted helminths (STHs) and not first‐line for schistosomiasis (which is typically treated with Praziquantel), the notion of “wholesale procurement” is relevant in broader helminth control programmes. Many endemic countries integrate mass-drug‐administration (MDA) not only for STHs but also for schistosomiasis and other neglected tropical diseases (NTDs). In such contexts:
Bulk procurement (wholesale) of relevant drugs ensures supply for large population-level treatment.
Even if mebendazole is not the primary schistosomiasis drug, countries may concurrently procure it wholesale for STHs while also coordinating praziquantel for schistosomiasis, especially given that environmental and sanitation interventions often address multiple helminth types.
Recognising that environmental interventions must accompany drug treatment means that budget planning for wholesale drug procurement must factor in that drugs alone will not suffice unless water/sanitation/environmental measures are in place.
Therefore, discussions around “mebendazole wholesale” fit into the larger public health strategy of helminth control, of which schistosomiasis is a key component and where environmental factors shape the success of the intervention.
Specific environmental factor linkages to spread
Here are concrete linkages
Temperature and snail population dynamics: Warmer temperatures within suitable range accelerate snail growth and schistosome development. According to the review, snail infection rates were around 0.5 when temperature conditions were favourable. Changing temperature regimes can extend transmission season or geographic range.
Rainfall, flooding and wetland expansion: Flooding creates or connects habitats favourable to snails; it also typically increases human exposure (displacement, reliance on alternative water sources). For instance, floods contributed to higher snail infection rates (0.5).
Water-resource projects: Irrigation canals, dams change hydrology: slow moving water or stagnant pools permit snail proliferation. This transforms formerly safe areas into risk zones. (Source: FAO environment/epidemiology document)
Sanitation and water-contact behaviour: Without sanitation, eggs enter water; children and women washing/clothes near water increases contact. The distribution is “highly focal” because water-contact behaviour drives exposure.
Vegetation, soil contamination and snail micro‐habitat: Aquatic vegetation offers snail cover; soil contamination with eggs may wash into water during rains. One study noted bacterial load and heavy‐metal concentrations in Schistosoma-positive samples, showing deeper environmental contamination links.
Challenges and emerging concerns
New transmission zones: “Previously uninfected areas, such as parts of Southeast Asia … are expected to become new hotspots due to changes in temperature and rainfall patterns.”
Focality of transmission: Control must be localised one village may have high risk due to a single water‐contact site with snails. General national averages may hide hotspots.
Intersectoral coordination: Water/sanitation, agriculture, hydrology, public health all need to align. Environmental changes intended for agriculture or energy (e.g., a dam) may increase schistosomiasis risk if snail habitat is created.
Sustained transmission due to environment: Even with repeated treatment, if human contact with infested water continues and snail habitat persists, reinfection occursthus making the environment a persistent risk.
What this means for policy and procurement (including wholesale drug supply)
Drug procurement planning (including mebendazole wholesale for STHs and praziquantel wholesale for schistosomiasis) must be matched with environmental interventions: Treating a community with drugs without reducing their exposure to infected water ensures rapid reinfection.
Environmental mapping and monitoring (water bodies, snail habitats, water‐contact sites) must inform where to target both environmental control and mass drug administration.
Wholesale procurement should consider the cyclical nature of transmission: if environment remains favourable, repeated treatments are needed, increasing demand for supplies.
Integrated helminth control programmes (combining schistosomiasis and STHs) may optimise procurement logistics wholesale ordering allows economies of scale.
Investment in sanitation, clean water supply, snail control, vegetation removal, ecological interventions (e.g. aquatic vegetation removal reduces snail habitat) can reduce the long‐term burden and hence reduce drug demand, making wholesale procurement more sustainable. (For example, one model showed vegetation removal improved health and increased agricultural productivity in a snail habitat area.)
Conclusions
The spread of Schistosoma infections is fundamentally shaped by environmental factors: freshwater habitat availability, snail intermediate‐host ecology, human water contact behaviour, sanitation, climate, and hydrology. Any control strategy that fails to address these will struggle to sustain success. From a procurement perspective, while wholesale drug supply (including mebendazole wholesale, when STHs are considered alongside Schistosoma) remains important, drugs alone are not enough. They must be embedded in an integrated environmental and public-health approach.
For practitioners, policymakers and procurement officers, the message is clear: to optimise drug use (and wholesale ordering), reduce environmental risk of exposure, map the hotspots carefully, and align environmental control with treatment cycles. This will reduce reinfection, enhance the cost‐effectiveness of wholesale drug supplies, and ultimately move communities closer to elimination of schistosomiasis and other helminthic infections.